LifeSign Made in USA
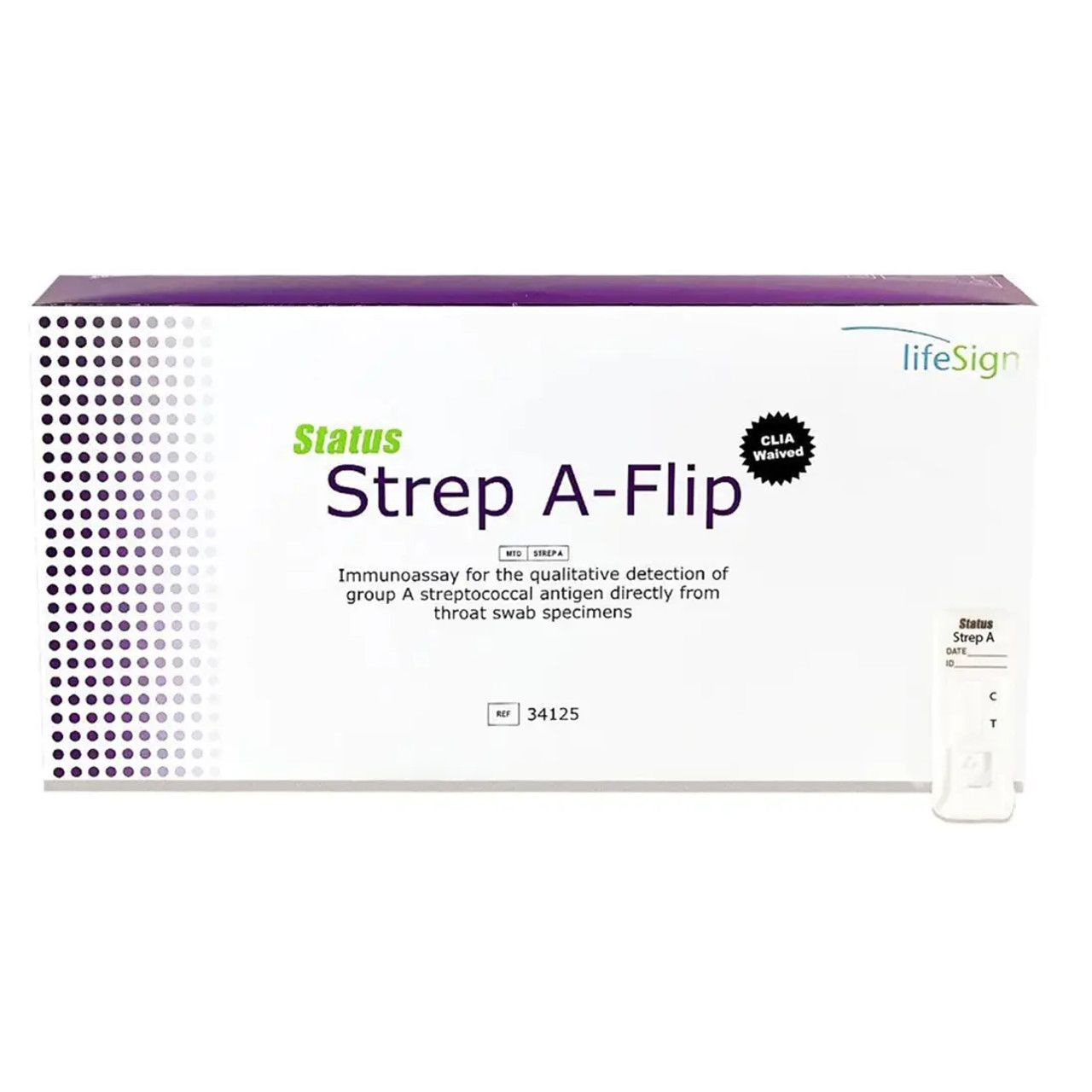
Strep A-Flip
+
Strep A-FLIP
Rapid Detection Of Group A Streptococcal Antigen Directly From Throat Swab Specimens
- CLIA WAIVED
- Single use reagent capsules
- Positive results in 5 min
- Sensitivity 96%, specificity 99%
- No counting reagent drops – fewer steps for greater accuracy
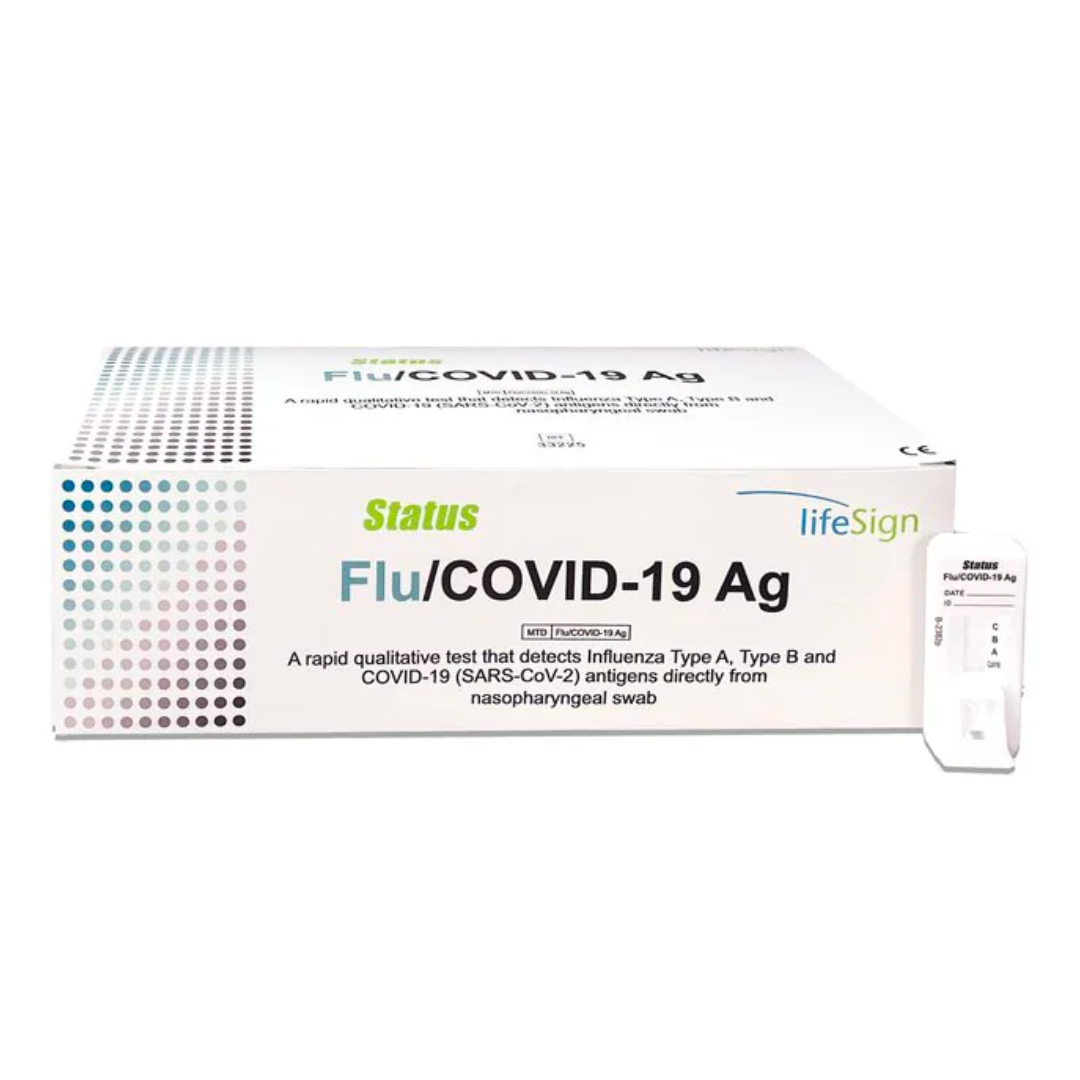
Flu/COVID-19 Ag
+
Flu/COVID-19 Ag A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from nasal or nasopharyngeal swab specimens
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked swab for superior specimen collection and patient comfort
- 25 tests per box
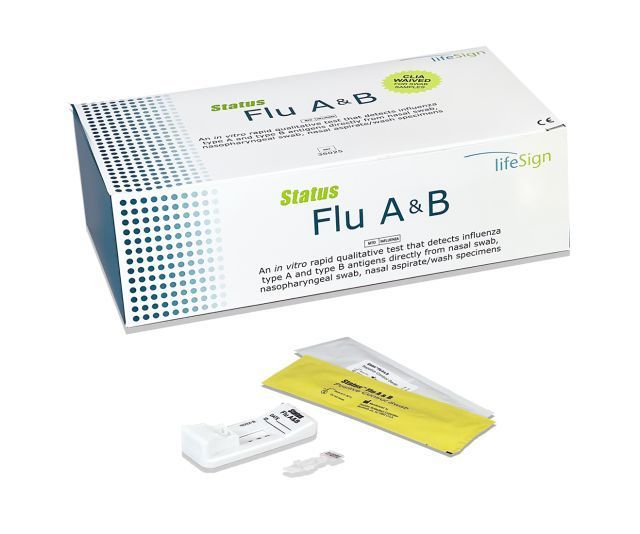
Status Flu A&B
+
Status™ Flu A&B, CLIA Waived, for nasal and NP swab specimens
- CLIA WAIVED for nasal swab and nasopharyngeal swab specimens
- Rapid diagnosis for early treatment
- 25 tests per box
- Innovative flip test design
- Easy-to-read cassette
- Pre-measured extraction reagent capsule for increased accuracy and ease of use
- Flocked nasal swabs included for better specimen collection
- Detects H1N1 (Swine Flu)
LifeSign MI TnI
+
LifeSign MI Troponin I
- Qualitative Troponin I
- Enables prompt emergency room decisions
- Easy to use procedure; Add 3 drops of specimen into device
- Read results in 15 minutes
- Improves patient care
- Reduces unnecessary testing and admissions
LifeSign MI TnI
+
LifeSign MI CK-MB/Myo/TnI
- MODERATELY COMPLEX
- Qualitative CK-MB, Myoglobin, Troponin I
- Enables prompt emergency room decisions
- Easy to use procedure; Add 3 drops of specimen into device
- Read results in 15 minutes
- Multiple panel assays
- Improves patient care
- Reduces unnecessary testing and admissions
LifeSign MI TnI
+
COVID-19/Flu
- MODERATELY COMPLEX
- Qualitative CK-MB, Myoglobin, Troponin I
- Enables prompt emergency room decisions
- Easy to use procedure; Add 3 drops of specimen into device
- Read results in 15 minutes
- Multiple panel assays
- Improves patient care
- Reduces unnecessary testing and admissions
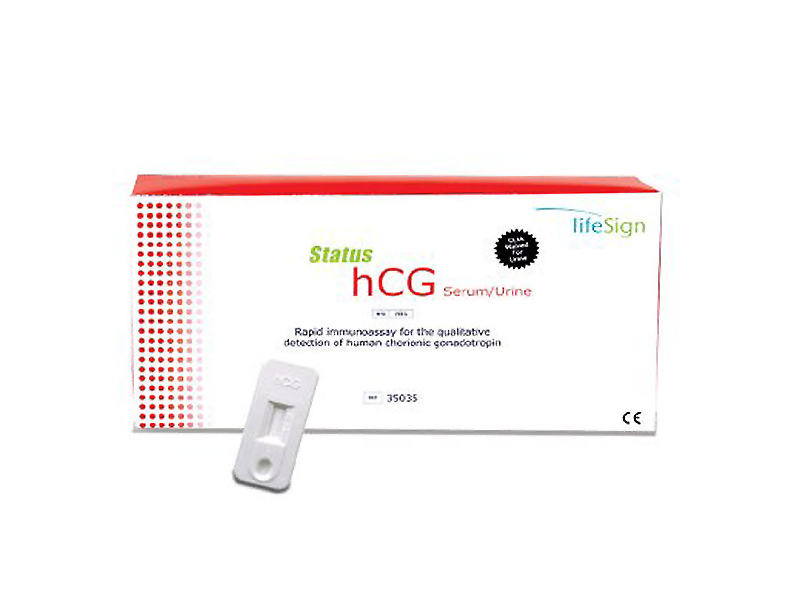
Status hCG
+
Status hCG serum/urine
- Designed to detect hCG in serum or urine
- CLIA waived for urine
- Serum and urine 25 mIU/mL sensitivity
- Physician’s licensing required. This is NOT an at-home test and should only be completed in a lab facility
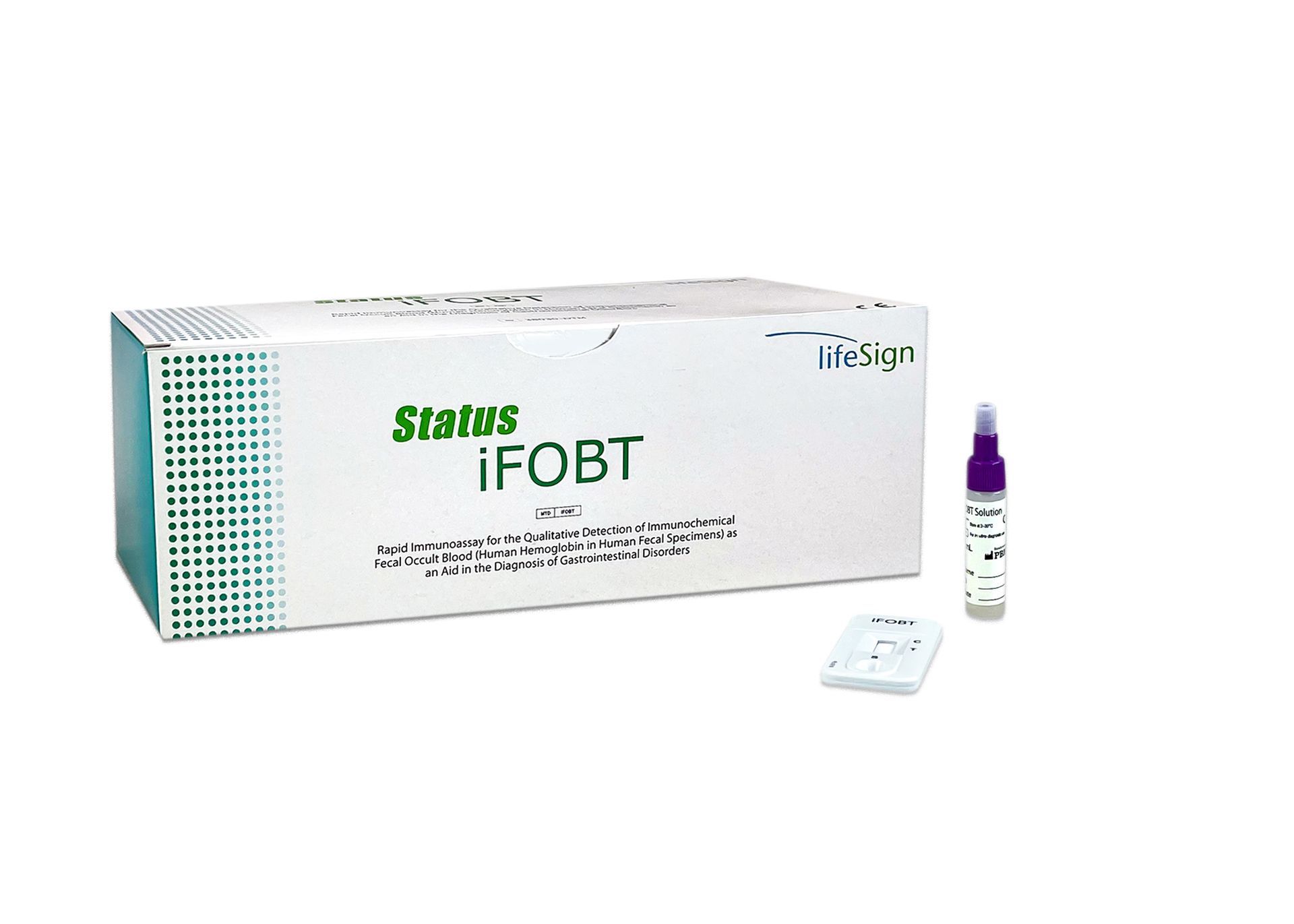
Status iFOBT
+
Status iFOBT
- CLIA Waived
- Superior sensitivity and specificity
- Human hemoglobin specific for reducing false positives
- Easy one-step test procedure
- Specimen can be collected in office or at home
- Patient friendly and easy-to-follow instructions
- Non-invasive collection method
- Only one test device needed
- No dietary or drug restrictions for iFOBT
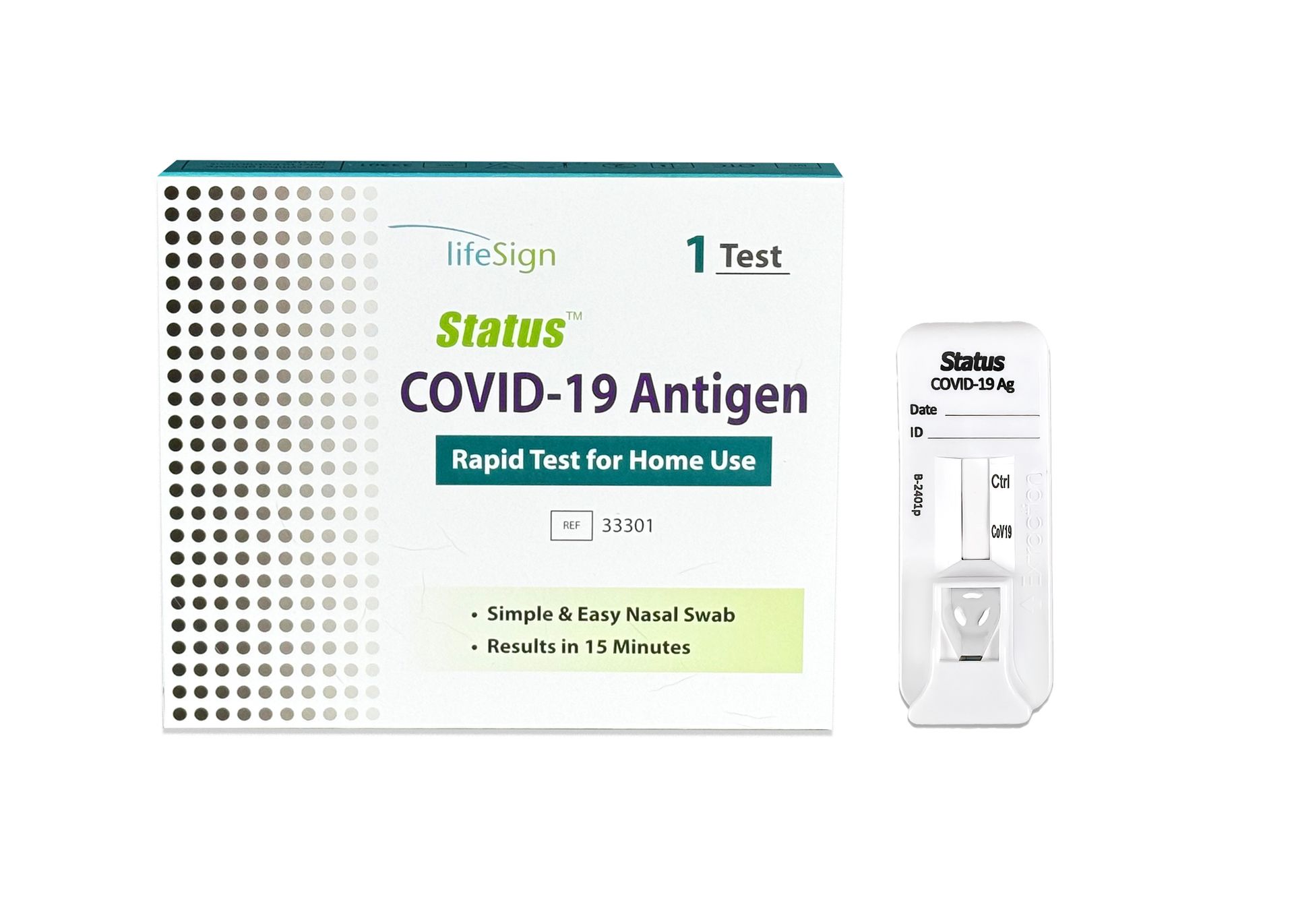
Status™ COVID-19 Antigen Rapid Test For Home Use
+
Status™ COVID-19 Antigen Rapid Test For Home Use (1)
- 1 Test per box
- FDA Emergency Use Authorization (EUA)
- Visually Read in 15 minutes
- For Home Use, Workplace Testing, Schools
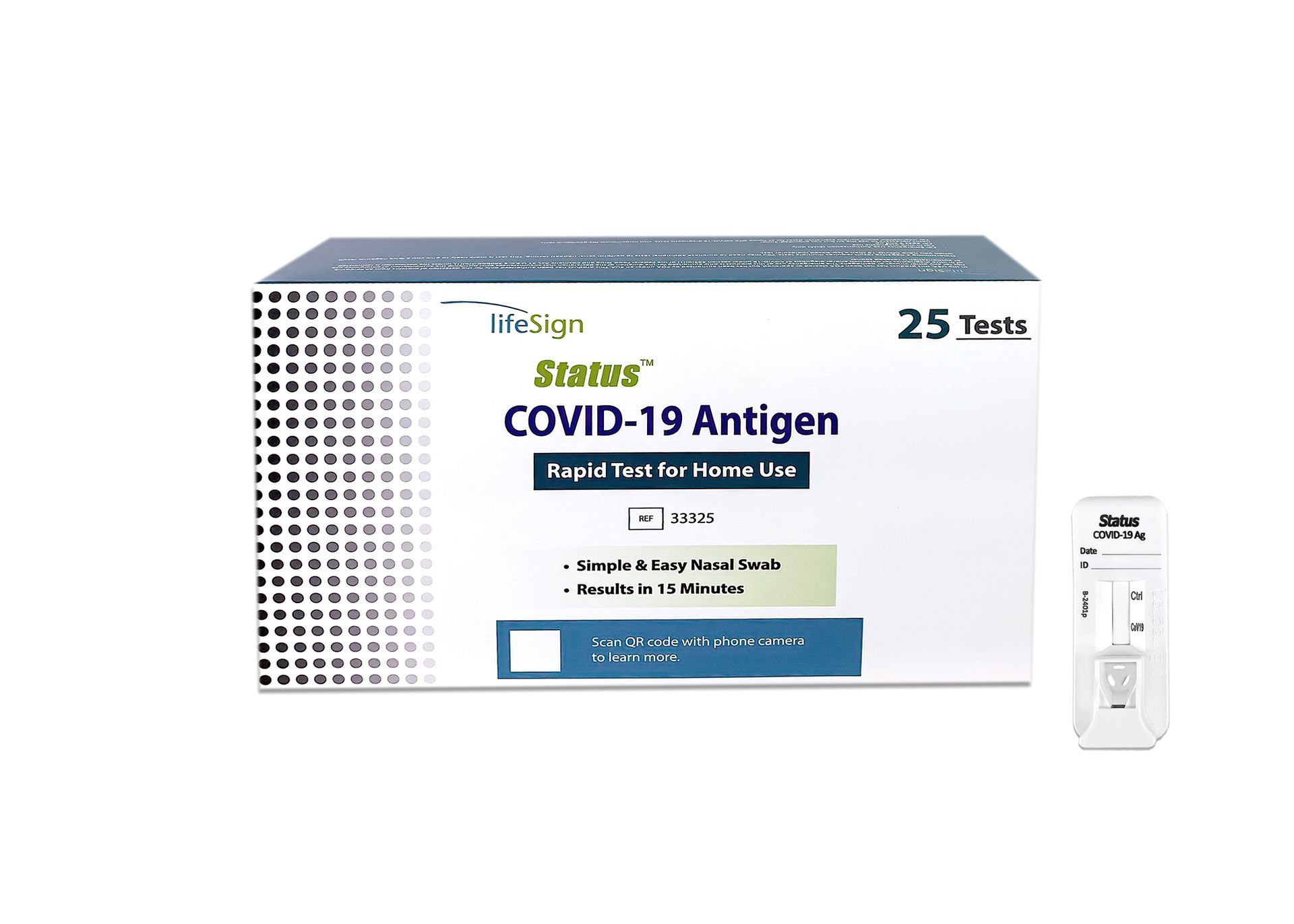
Status™ COVID-19 Antigen Rapid Test For Home Use
+
Status™ COVID-19 Antigen Rapid Test For Home Use (25)
- 25 Tests per box
- FDA Emergency Use Authorization (EUA)
- Visually Read in 15 minutes
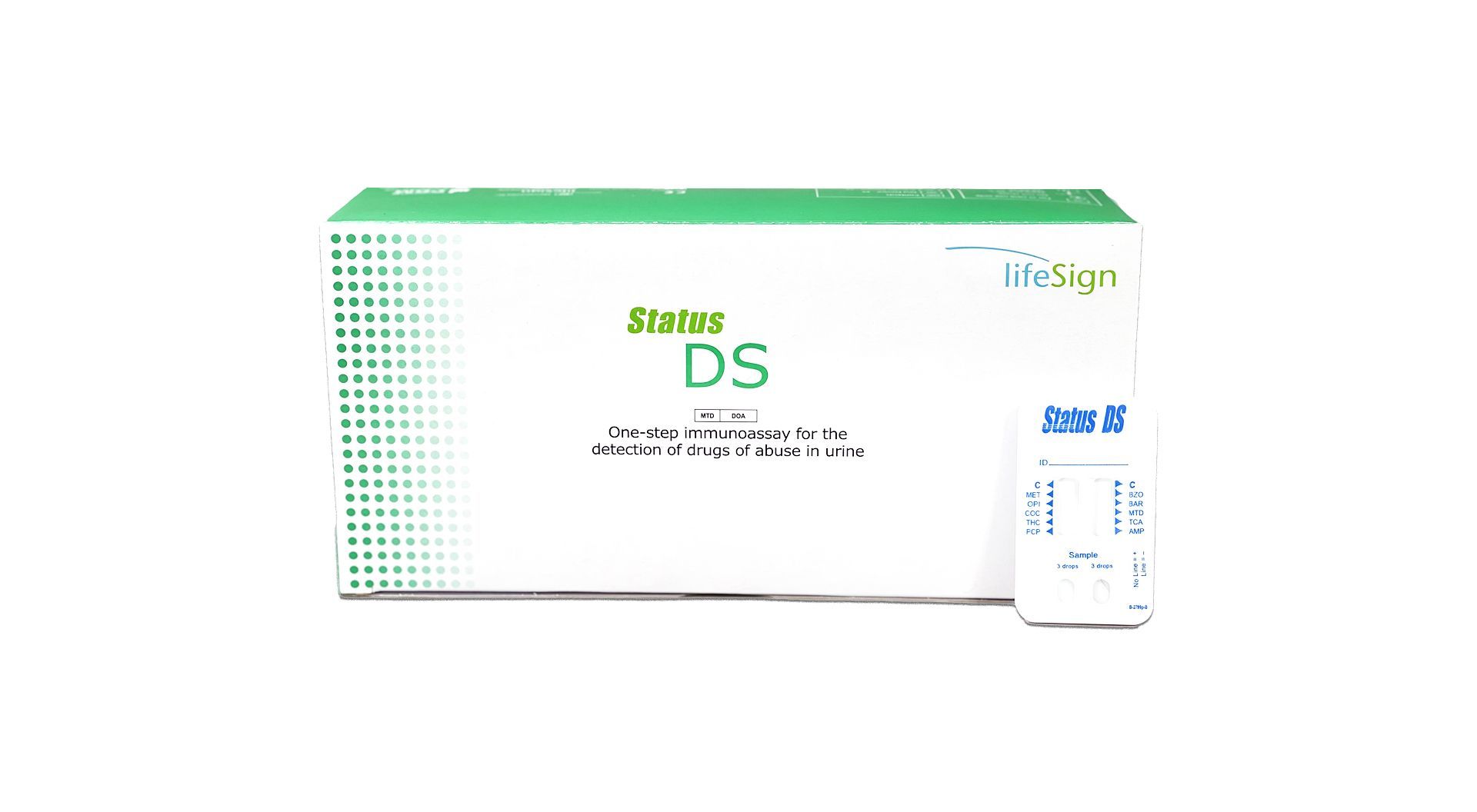
Status DS
+
Status DS
- 25 Tests per box
- Simple one-step procedure
- Accurate results with a 97-99% correlation to GC/MS
- Fast turnaround time with results in 5-10 minutes
Status MONO
+
Status MONO
- CLIA WAIVED for whole blood
- FDA cleared with no age restrictions
- Single reagent procedure provides results in 3-8 minutes
- Fingerstick whole blood convenience
- Superior 99.9% sensitivity for earlier IM detection, specificity of 98.8%
- Multiple kit sizes available, ideal for any practice
- External QC negative and positive controls included (item # 68364 & 84M30)
- New plastic sample transfer pipettes
Status Nicotine
+
Status Nicotine
- Simple one-step procedure
- Results in as little as 5-10 minutes
- 500 ng/mL cut-off detects active nicotine intake
- Useful in immediate counseling and intervention
Uricult (Urinary Tract Infections)
+
Uricult (Urinary Tract Infections)
- Moderately Complex
- Culture method for urinary pathogens
- Easy to perform procedure; Dip - Incubate - Read Results
- Quick and convenient result within 24 hours
- Simple CLIA compliance for reporting colony counts.
Alco-Screen is a rapid, highly sensitive method to detect the presence of alcohol in saliva and provide an approximation of relative blood alcohol concentration. Since it is well established that the concentration of alcohol in saliva is very close to that in the blood, saliva is the preferred specimen for alcohol testing, allowing for greater detection sensitivity than breath testing methods. Alco-Screen is a very simple, one-step, 2 minute test requiring no instrumentation, calibration or special training to be used effectively, and is convenient enough to be used any time, any place.
Alco-Screen Saliva Alcohol Test
Testing with the Alco-Screen saliva alcohol test is fast and easy. The operator has the subject spit into a cup and dips the reagant tip of the strip into the saliva for several seconds. The Alco-Screen requires such a small amount of saliva to activate the test, even subjects with dry mouth can be tested successfully. Results are visible within two minutes after wetting the test strip with saliva.
Alco-Screen Estimates Alcohol Concentration
Because the proportion of alcohol in saliva is directly related to the proportion of alcohol in blood, the Alco-Screen can easily and quickly detect the presence of alcohol and estimate intoxication levels. The presence of alcohol in saliva causes the reagent pad to turn shades of green. Higher concentrations of alcohol create darker shades of green. The operator estimates intoxication levels by comparing the color change against color standards printed on the foil package at the 0.02%, 0.04%, 0.08%, and 0.30% BAC.
Ideal for Zero Tolerance Testing
The Alco-Screen is ideal for agencies with zero tolerance policies and for non-regulated employee workplace testing programs. Any green color on the reagent pad after two minutes indicates the presence of alcohol in the saliva of at least 0.02% or greater. 0.02% BAC is approximately the intoxication level from one drink, and is typically the lowest cut-off level used to determine if a person has been drinking alcohol.
Alco-Screen Detects Alcohol in Beverages
The Alco-Screen is one of the few devices that can detect the presence of alcohol in beverages. Because the Alco-Screen is calibrated to detect the very tiny amounts of alcohol present in saliva, the color standards on the test do not apply when testing beverages. The concentration of alcohol is extremely high in comparison to saliva. Dipping the strip into a beverage that contains alcohol causes the reagent pad to turn a very dark brown color.
STEP 1 – Collect a subject’s saliva in a clean, unused cup and dip the reagent end of test strip in the cup for several seconds. Once the reagant pad is saturated with the saliva, place the strip on a flat surface and set a 2 minute timer.
STEP 2 – After 2 minutes compare the color change against the color standards on the back of the envelope package to estimate alcohol level.
On-Site Testing Specialists provides this exam and certification to evaluate the knowledge of an individual in regards to specimen collection, device testing and test interpretation according to the manufacturers recommendations. This certification is NOT an accredited certification for DOT or workplace specimen collection/testing.
ALCO Screen 2
+
Quick 4 Minute On-Site Test for Alcohol
- Convenient use anywhere anytime
- FDA Cleared
- DOT Approved
- CLIA Waived
- Over the counter use
- 24 tests per box
Alco-Screen® 02 is a simple, cost effective tool used to monitor alcohol consumption as part of a comprehensive Zero Tolerance testing program. Alco-Screen® 02 is a Conforming Product for US Department of Transportation mandated testing of all transportation and safety sensitive employees for blood alcohol concentrations above the federally mandated zero tolerance level of 0.02%. Alco-Screen® 02 is FDA 510(k) cleared for home use and is CLIA waived.
Convenient, Fast Results
Testing with the Alco-Screen® 02 is as easy as placing one end of the test strip in the subjects mouth for a few seconds to wet it with saliva. The operator reads results in four minutes.
Estimates Alcohol Concentration
Because the proportion of alcohol in saliva is directly related to the proportion of alcohol in blood, the Alco-Screen® 02 can easily and quickly detect the presence of alcohol and estimate intoxication levels. The presence of alcohol in saliva causes the reagent pad to turn shades of green. Higher concentrations of alcohol create darker shades of green. The operator estimates intoxication levels by comparing the color change against color standards printed on the foil package at the 0.02%, 0.04%, 0.08%, and 0.30% BAC.
Ideal for Zero Tolerance Testing
The Alco-Screen® 02 is ideal for agencies with zero tolerance policies. Any green color on the reagent pad after two minutes indicates the presence of alcohol of at least 0.02% or greater. 0.02% BAC is about the intoxication from one drink and is typically the lowest cut-off level used to determine if a person has been drinking alcohol.
Detects Alcohol in Beverages
The Alco-Screen® 02 is one of the few devices that can detect the presence of alcohol in beverages. Because the Alco-Screen is calibrated to detect the very tiny amounts of alcohol present in saliva, the color standards on the test do not apply when testing beverages. The concentration of alcohol is extremely high in comparison to saliva. Dipping the strip into a beverage that contains alcohol causes the reagent pad to turn a very dark brown color.
Alco-Screen® 02 is a one-step saliva screening test that is minimally invasive and provides results within 4 minutes. Simply wet the test pad with saliva; development of a distinct colored line on the test pad within 4 minutes indicates a blood alcohol concentration equal to or exceeding 0.02%
DOT Approved as Alcohol Screening Device
The Alco-Screen® 02 was one of the first devices to be approved by the U.S. Department of Transportation (DOT) as an Alcohol Screening Device. DOT approval means that the Alco-Screen reliably and accurately detects alcohol at the 0.02 concentration or higher and does not give false positive readings when no alcohol is present. DOT approval allows users to use the Alco-Screen® 02 for screening tests required by Dept. of Transportation regulations.
On-Site Testing Specialists provides this exam and certification to evaluate the knowledge of an individual in regards to specimen collection, device testing and test interpretation according to the manufacturers recommendations. This certification is NOT an accredited certification for DOT or workplace specimen collection/testing.
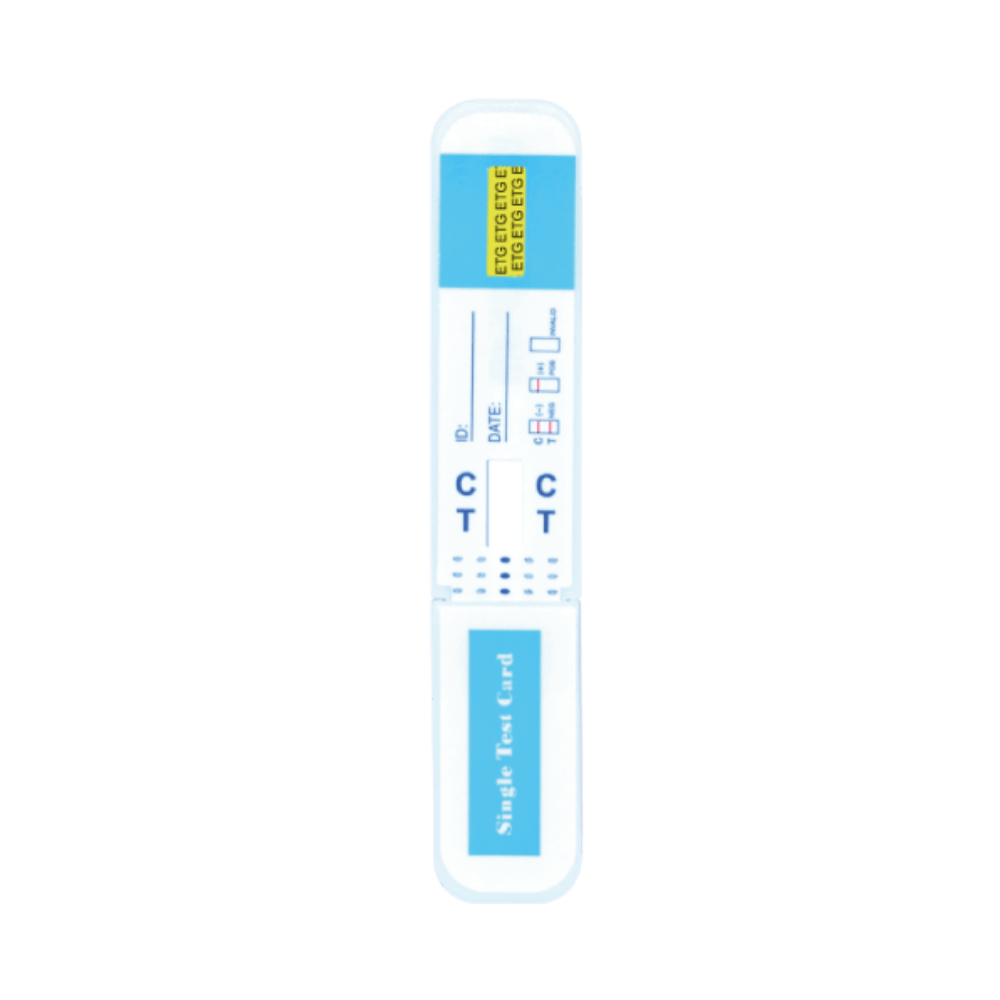
ETG Test
+
ETG Alcohol Urine Tests
- 25 Urine alcohol test kits for Ethyl Glucuronide (EtG)
- Up to 80 hour detection time!
- 12-18 month expiration date on all urine drug test cards (guaranteed)
- Fast results in 5 minutes
The One Step Drug of Abuse Test is a competitive immunoassay utilizing highly specific reactions
between antibodies and antigens for the detection of multiple drugs and drug metabolites in
human urine.
The One Step Drug of Abuse Test is a rapid urine screening test that utilizes monoclonal
antibodies to selectively detect elevated levels of specific drugs in urine without the use of an
instrument.
The One Step Drug of Abuse Test is an immunoassay based on the principle of competitive
binding. Drugs which may be present in the urine specimen compete against their respective drug
conjugate for binding sites on their specific antibody.
During testing, a urine specimen migrates upward by capillary action. A drug, if present in the urine
specimen below its cut-off concentration, will not saturate the binding sites of its specific antibody.
The antibody will then react with the drug-protein conjugate and a visible colored line will show up
in the test line region of the specific drug strip. The presence of drug above the cut-off
concentration will saturate all the binding sites of the antibody. Therefore, the colored line will not
form in the test line region.
A drug-positive urine specimen will not generate a colored line in the specific test line region of the
strip because of drug competition, while a drug-negative urine specimen will generate a line in the
test line region because of the absence of drug competition.
To serve as a procedural control, a colored line will always appear at the control line region,
indicating that proper volume of specimen has been added and membrane wicking has occurred.
Allow the test device and urine speimen to come to room temperature [15-30oC (59-86oF)] prior to
testing.
[For Strip]
1) Remove the strip from the foil wrapper or the desiccated container (bring the container to the room
temperature before opening to avoid condensation of moisture in container). Label the strip with patient
or control identifications.
2) Immerse the strip into the urine with the arrow end pointing toward the urine. Do not cover the urine
over the MAX (maximum) line. You may leave the strip in the urine or you may take the strip out after a
minimum of 15 seconds in the urine and lay the strip flatly on a non-absorptive clean surface.
3) Read results at 5 minutes.
DO NOT INTERPRET RESULT AFTER 10 MINUTES.
[For Cassette]
1) Remove the test device from its foil wrapper by tearing along the slice (bring the container to the room
temperature before opening to avoid condensation of moisture in container). Label the device with
patient or control identifications.
2) Using the specimen dropper, withdraw the urine sample from the specimen cup and slowly dispense 3
drops (approximately 120uL) into the circular sample well, being careful not to overfill the absorbent
pad.
3) Read results at 5 minutes. DO NOT INTERPRET RESULT AFTER 10 MINUTES.
[For Dipcard]
1) Remove the test device from the foil pouch.
2) Remove the cap from the test device. Label the device with patient or control identifications.
3) Immerse the absorbent tip into the urine sample for 5 seconds. Urine sample should not touch the
plastic device.
4) Replace the cap over the absorbent tip and lay the device flatly on a non-absorptive clean surface.
5) Read results at 5 minutes.DO NOT INTERPRET RESULT AFTER 10 MINUTES.
[For Multi-Drug Screen Test Cup]
Please follow the instructions on the Procedure CardAlco-Screen® 02 is a one-step saliva screening test that is minimally invasive and provides results within 4 minutes. Simply wet the test pad with saliva; development of a distinct colored line on the test pad within 4 minutes indicates a blood alcohol concentration equal to or exceeding 0.02%
DOT Approved as Alcohol Screening Device
The Alco-Screen® 02 was one of the first devices to be approved by the U.S. Department of Transportation (DOT) as an Alcohol Screening Device. DOT approval means that the Alco-Screen reliably and accurately detects alcohol at the 0.02 concentration or higher and does not give false positive readings when no alcohol is present. DOT approval allows users to use the Alco-Screen® 02 for screening tests required by Dept. of Transportation regulations.
Contact Us
We will get back to you as soon as possible.
Please try again later.
All Rights Reserved | On-Site Testing Specialists